| Title | Calcium binding peptide motifs from calmodulin confer divalent ion selectivity to elastin-like polypeptides |
| Publication Type | Journal Article |
| Year of Publication | 2013 |
| Authors | Hassouneh, W, Nunalee, ML, Shelton, CM, Chilkoti, A |
| Journal | Biomacromolecules |
| Volume | 14 |
| Issue | 7 |
| Pagination | 2347–2353 |
| Date Published | 05/2013 |
| ISSN | 1526-4602 |
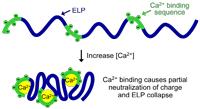
| Abstract | Calcium sensitive elastin-like polypeptides (CELPs) were synthesized by periodically interspersing a calcium-binding peptide sequence from calmodulin within an elastin-like polypeptide (ELP), with the goal of creating thermal and calcium responsive peptide polymers. The CELPs exhibit high sensitivity to calcium compared to monovalent cations but do not exhibit the exquisite selectivity for calcium over other divalent cations such as magnesium that is displayed by calmodulin. The CELPs were further used as a building block for the synthesis of calcium sensitive nanoparticles by fusing a hydrophilic, non-calcium sensitive ELP block with a CELP block that becomes more hydrophobic upon calcium binding. We show that addition of calcium at concentrations between 50–500 mM imparts sufficient amphiphilicity to the diblock polypeptide between 33 and 46 °C to trigger its self-assembly into monodisperse spherical micelles with a hydrodynamic radius of ~50 nm. |
| DOI | 10.1021/bm400464s |
| Short Title | Biomacromolecules |