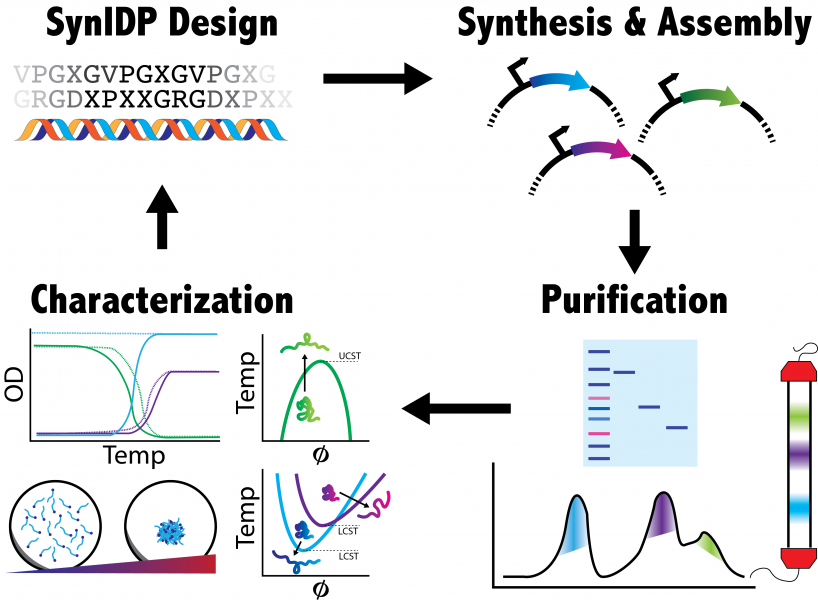
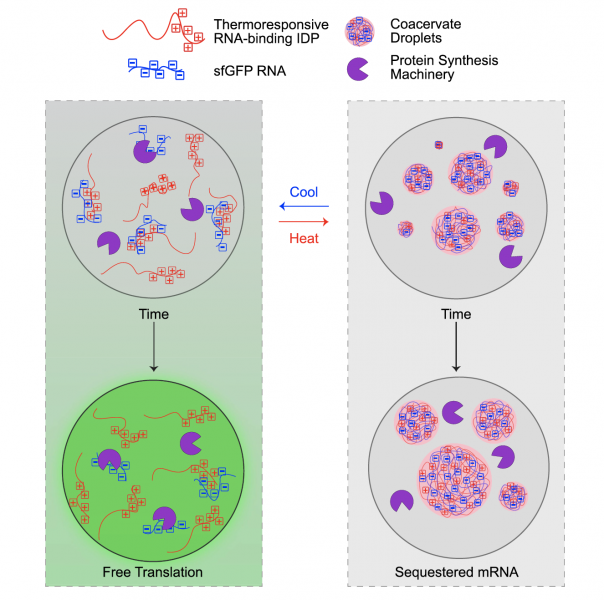
Intrinsically disordered proteins (IDPs) are important components in the cellular function and their biological role has been the focus of a growing number of publications. “Liquid-like" granules rich in intrinsically disordered proteins and RNA play key roles in critical cellular functions such as RNA processing and translation. These complexes or RNA and RNA-binding proteins, known as Ribonucleoproteins (RNP), are commonly involved in processing RNA transcripts and mediate cell function in response to stress and other environmental perturbations. The malfunction of these granules has been implicated in numerous diseases, however the mechanistic underpinnings of these disease states is not well understood. Elastin-like polypeptides (ELPs) are a family of lab-designed IDPs which, while not normally found in nature, share many of the same characteristics as natural IDPs including temperature responsiveness and salt/pH sensitivity. Since ELPs are genetically encoded as a sequence of amino acids, they can be fused to various RNA binding proteins and form synthetic IDP-RNA-rich granules. These synthetic granules can be engineered for a variety of synthetic biology applications. For example, one can recreate natural IDP-RNA granules using synthetic IDP-RNA granules to study the mechanistic underpinnings of natural systems and their disease states. From a genetic engineering standpoint, one can use ELPs fused to a protein targeting specific mRNAs to modulate gene expression by sequestering target mRNA using ELPs' temperature-responsive phase separation activity.
An Ode to IDP's, by Daniel Shapiro:
IDP intrinsically disordered
IDP sway
IDP protein
IDP binds RNA
IDP clump
IDP sequester
IDP hides RNA
Ribosome can’t get ‘er
IDP wiggle
IDP repeat
IDP jiggle
IDP VPGVGeet
IDP turn gene off
IDP inhibit
IDP doesn’t work as expected
IDP a whole clown exhibit
IDP at 20 degrees
IDP soluble
IDP at 37 degrees?
IDP insoluble
IDP is love
IDP is life
IDP doesn’t purify
IDP causes me strife
IDP I love you
IDP is for me
IDP controls gene expression
IDP makes my PhD