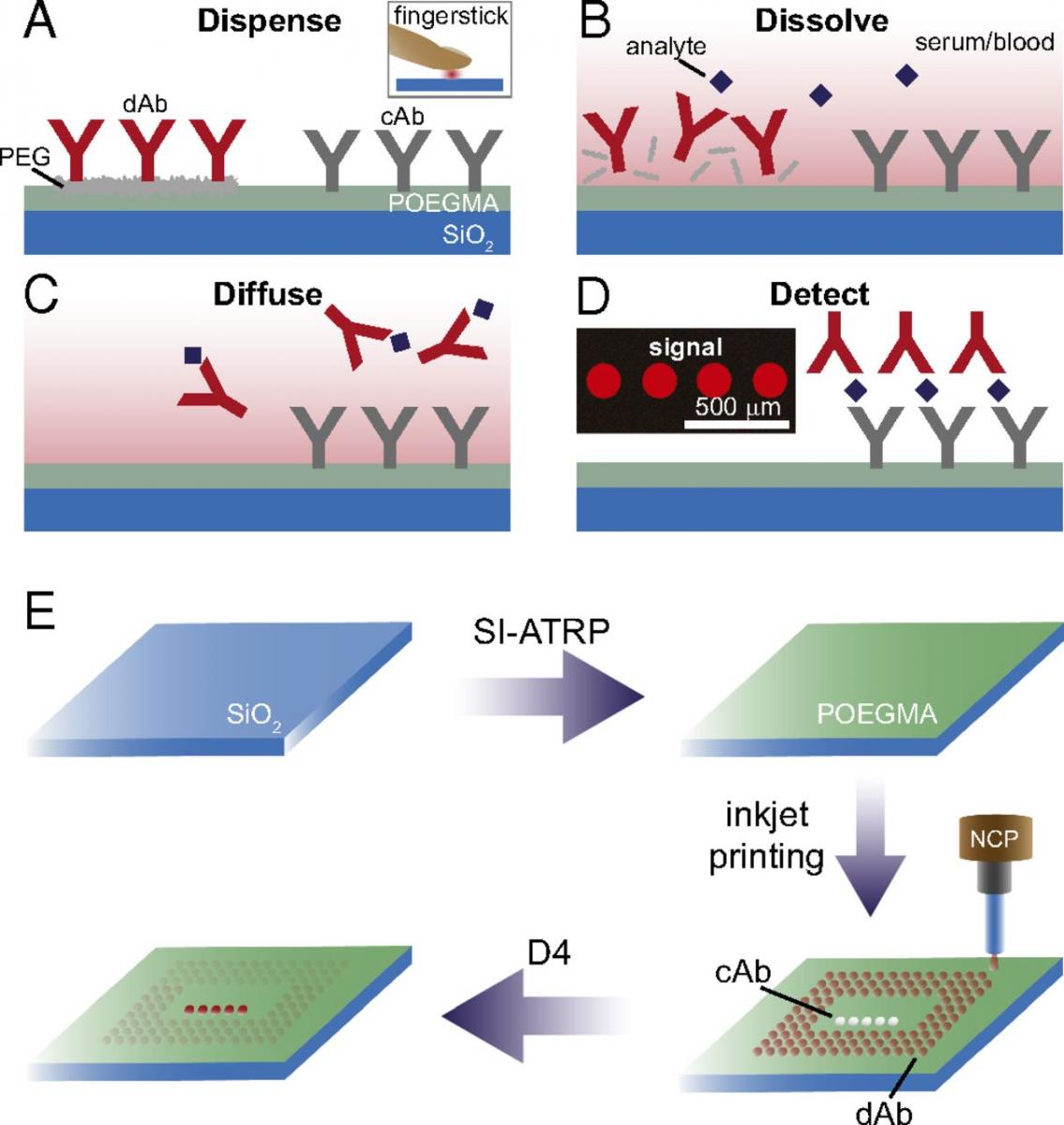
The D4 assay is a point-of-care diagnostic that we have developed, in which all reagents are printed and stored on a “non-fouling”—protein and cell resistant—polymer brush. The D4 assay, involves four sequential events: (1) Dispense (droplet of blood); (2) Dissolve (printed reagents on chip); (3) Diffuse (across surface); and (4) Detect (binding event). The D4 POCT antibody (Ab) consists of microarrays printed on the polymer brush yields quantitative results, with picomolar sensitivity within 30 minutes. All reagents are inkjet-printed and stored on D4 POCT cassettes, which do not require refrigeration. Upon direct application of fingerstick blood onto a cassette, analyte capture and detection occur automatically, generating a quantifiable fluorescence signal obtained by placing the cassette in a small device that magnetically attaches to a smart phone, which images and analyzes microarrays via on-board App. The D4 assay can be used for the diagnosis of all markers for which antibody pairs are available with a speed and sensitivity that is as good or better than commercially available point-of-care tests and is far simpler, cheaper more rugged, and does not require a cold-chain.
We are currently developing multiplexed POCTs based on the D4 platform for the following diseases:
Global Health: The disease burden in the developing world is still largely dominated by infectious diseases such as malaria, HIV, and re-emerging diseases like Ebola. The availability of rapid and accurate point of care diagnostics is vital in reducing the burden of these diseases. Our lab is developing rapid point of care diagnostic devices targeting the most prevalent diseases in the developing world. By combining three technologies developed in our lab: (1) the D4 assay, (2) self-contained microfluidic platforms and (3) the D4Acope, we are creating point-of-care tests with high (femtomolar) sensitivity, that are low-cost and robust and hence useful for low resource settings for global health applications.
Cancer: We are developing a multiplexed D4 POCT for detection of several serum biomarkers of hepatocellular carcinoma from a single drop of blood. We are currently in the process of validating the test and biomarkers. If successful, this technology will assist in cancer screening programs, particularly in lower-resource settings where incidence and mortality are highest.
Infectious Diseases: We have several active projects that are aiming to developing rapid POCTs for infectious disease (Malaria, Ebola, HIV, Zika, Talaromycosis) screening in low-resource settings.